Sample Business Contracts
Supply Agreement - Merisant Co., Merisant Co. 1 Sarl and the NutraSweet Co.
Free Supply Agreement Forms
Sponsored Links
CONFIDENTIAL MATERIAL APPEARING IN THIS DOCUMENT WAS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION IN ACCORDANCE WITH RULE 24b-2, PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. OMITTED INFORMATION WAS REPLACED WITH ASTERISKS.
SECOND AMENDED AND RESTATED SUPPLY AGREEMENT
THIS SECOND AMENDED AND RESTATED SUPPLY AGREEMENT (this "Agreement") is made as of this 31st day of December, 2003, by and between Merisant Company, a Delaware corporation, having its principal place of business at 10 S. Riverside Plaza, Chicago, IL 60606 ("Merisant"), Merisant Company 1 Sarl, a company organized under the laws of Switzerland, having its principal place of business at Avenue J. J. Rousseau 7, 2000 Nechatel, Switzerland ("Swissco" and, together with Merisant, "Buyer"), and The NutraSweet Company, a Delaware corporation, having its principal place of business at 200 World Trade Center, The Merchandise Mart, Suite 936, Chicago, IL 60654 ("NSC").
RECITALS:
WHEREAS, Buyer, as successor to Tabletop Sweetener, LP, and NSC, as successor to Monsanto Company, are parties to that certain Amended and Restated Supply Agreement, dated February 3, 2000, as amended and supplemented by that certain First Addendum to Amended and Restated Supply Agreement, dated November 1, 2002, and further amended and supplemented by that certain Second Addendum to Amended and Restated Supply Agreement, dated July 17, 2003 (collectively, the "Original Agreement"), pursuant to which NSC has agreed to supply Buyer's requirements for aspartame for use in the manufacture, marketing, distribution and sale of tabletop sweetener products to consumer end-users and establishments serving or selling to such consumer end-users (including any successor thereto, the "Business") through December 31, 2004.
WHEREAS, certain affiliates and designees of Merisant have been purchasing from NSC under the Original Agreement;
WHEREAS, Merisant and NSC desire to add Swissco as a party to this Agreement to clarify the purchasing arrangements hereunder;
WHEREAS, Buyer and NSC have agreed that, subject to the terms and conditions and during the term set forth herein, NSC will sell to Buyer, and Buyer will buy from NSC, aspartame for continued use in the Business on the amended and restated terms set forth herein.
THE PARTIES HAVE AGREED:
1. Supply of aspartame. During the Term (as defined below), NSC agrees to sell and cause its affiliates to sell, and Buyer agrees to buy, or cause the Business to buy (through Buyer's Affiliates or designees), aspartame manufactured in accordance with the specifications set forth in Exhibit A attached hereto ("Powder Product") and, solely during calendar year 2005, aspartame manufactured in accordance with the specifications set forth in Exhibit B attached hereto ("Fine Granular Product" and, together with the Powder Product, the "Product") (the specifications set forth on Exhibits A and B referred to herein as the "Specifications").
2. Term. Unless earlier terminated as provided in paragraph 4 of Exhibit C attached hereto or in this Section 2, this Agreement shall be in effect for the period commencing on the date hereof and ending on December 31, 2005 (the "Term").
3. Purchase/Use.
(a) During the Term of this Agreement, subject to the terms hereof, Buyer agrees to buy, or cause the Business to buy, from NSC, and NSC agrees to sell or cause to be sold to Buyer or the Business, the number of pounds of Product set forth on Exhibit D. During calendar year 2004, NSC shall be the Buyer's exclusive supplier of aspartame for use in tabletop sweetener products
manufactured by or for Buyer and its Affiliates for sale in the Territory (as defined below), and Buyer shall not purchase aspartame from any other person for use in such products. Product purchased under the terms of this Agreement may be used in tabletop sweetener products manufactured by Buyer and its Affiliates that are sold only in those areas of the world, set forth in Exhibit D (the "Territory"). Notwithstanding the foregoing, if NSC is unable, because of legal, regulatory or contractual constraints, or otherwise fails, to supply a sufficient amount of Product to Buyer to meet its requirements in the Territory, then Buyer shall be permitted to buy Product from a third-party supplier for use in the Territory for so long as such inability or failure exists.
(b) Upon execution of this Agreement, and thereafter at least sixty (60) days prior to the commencement of each succeeding calendar quarter during the Term, Buyer shall provide NSC with a non-binding forecast of the quantity of Product that Buyer estimates the Business will require in the Territory for the immediately succeeding calendar quarter and the next succeeding three (3) calendar quarters. At least thirty (30) days prior to the commencement of each calendar month during the Term, Buyer shall provide NSC with a firm order for the quantities of Product the Business will require in the Territory during such calendar month, and NSC will timely fill such order. During the Term, Buyer and NSC shall meet as regularly as necessary to discuss the schedule of production and deliver so as to ensure that all quantities agreed to for each calendar year are ordered and delivered in a predictable, orderly manner throughout such year.
4. Price. The prices for Product during the Term are set forth on Exhibit D attached hereto.
5. Terms and Conditions. This Agreement shall also be governed by the Other Terms and Conditions set forth on Exhibit C attached hereto. In the event of any conflict or inconsistency between the other terms and conditions set forth on Exhibit C attached hereto and the terms and conditions within the body of this Agreement, the terms and conditions within the body of this Agreement shall control.
6. License of Use of NutraSweet Logo on Packaging. Buyer acknowledges that it shall have no rights to any "NutraSweet" trademarks or "Swirl" designs other than as expressly set forth in (a) the Amended and Restated Trademark License Agreement, dated March 17, 2000, by and between NSC and Tablesweet Inc. and (b) the Amended and Restated Trademark Sub-License Agreement, dated March 17, 2000, by and between Tablesweet Inc. and Tabletop Sweetener, L.P.
7. Confidentiality. (a) Except, upon prior notice to the other party, to a governmental agency in response to any written demand by such agency pursuant to applicable law or to governmental health authorities to obtain and maintain the registration of aspartame, a party receiving confidential information hereunder shall not, without the specific written consent of the other party, disclose to any third party or use for its own purposes any information which is received from the disclosing party, including, without limitation, the price and similar terms of this Agreement, unless such information:
(i) is or becomes generally available to the public other than through the action of the receive party, its agents or employees;
(ii) was, at the time of receipt by the receiving party, already in the receiving party's possession as evidenced by its written records; or
(iii) was obtained by the receiving party from a third party legally entitled to use and disclose the same.
Notwithstanding the foregoing, (1) Buyer or its Affiliates may disclose this Agreement and any of the terms (other than volume, price and territory information set out on Exhibit D) hereunder (A) as required by the Indenture dated as of July 11, 2003 by and among Merisant Company, the guarantors from time to time a party thereto and Wells Fargo Bank Minnesota, National Association, as Trustee; (B) as required by the Indenture dated as of November11, 2003 by and between Tabletop
2
Holdings, Inc. and Wells Fargo Bank Minnesota, National Association, as Trustee; and (C) as required by the Securities Act of 1933 (the "Securities Act") or the Securities Exchange Act of 1934 (the "Exchange Act") and the rules and regulations promulgated thereunder; provided that any description of this Agreement included in any document provided to any third party is subject to the prior approval of the other party, which approval will not be unreasonably delayed or withheld; and (2) either party hereto may disclose this Agreement and any of the terms hereunder (A) to a potential buyer in connection with the sale, directly or indirectly, of all or substantially all of such party's business, or (B) to any creditor or prospective creditor of such party or its Affiliates (or any agent on behalf thereof) in connection with such creditor's or prospective creditor's due diligence and/or review of such party's or such Affiliate's books and records.
(b) Notwithstanding anything herein to the contrary, confidential information shall not include, and each of the parties and their respective Affiliates (and the respective partners, directors, officers, employees, advisors, representatives and other agents of each of the foregoing and their Affiliates) may disclose to any and all persons, without limitation of any kind, (i) any information with respect to the U.S. federal and state income tax treatment of the transactions contemplated hereby and any facts that may be relevant to understanding such tax treatment, which facts shall not include for this purpose the names of the parties or any other person named herein, or information that would permit identification of the parties or such other persons, or any pricing terms or other nonpublic business or financial information that is unrelated to such tax treatment or facts, and (ii) all materials of any kind (including opinions or other tax analyses) relating to such tax treatment or facts that are provided to any of the persons referred to above, and it is hereby confirmed that each of the persons referred to above has been authorized to make such disclosures since the commencement of discussions regarding the transactions contemplated hereby.
8. Release. Each of the parties hereto does for itself and on behalf of each of its subsidiaries, representatives, officers, directors, employees, agents, Affiliates, predecessors, successors and/or assigns, hereby release, remise and forever discharge the other party hereto and each of such party's respective present or former subsidiaries, representatives, officers, directors, employees, agents, Affiliates, predecessors, successors and/or assigns from any and all claims, charges, controversies, covenants, rights, promises, trespasses, damages, losses and expenses, debts, dues, demands, sums of money, actions, rights, causes of action, obligations and liabilities of any kind or nature whatsoever, at law or in equity, whether asserted or unasserted, mature or contingent, know or unknown, accrued or unaccrued, which such releasing party may have had, claims to have had, now has, may claim to have or claims to have, which are or may be based upon any facts, acts, conduct, representations, omissions, contracts, claims, events, causes, matters or things of any conceivable kind or character arising out of or relating to the Original Agreement, arising on or prior to the date of this Agreement. Notwithstanding the foregoing, Buyer shall continue to be obligated to pay for Product delivered by Supplier prior to the date of this Agreement in compliance with the terms of the Original Agreement.
9. Entire Agreement. This Agreement (including Exhibits attached hereto) constitutes the entire agreement between the parties relating to the supply of Product by NSC to Buyer. All prior agreements or arrangements between the parties relating to the supply of aspartame, whether written or oral, including the Original Agreement and that certain agreement dated November 1, 2002 pursuant to which Buyer agreed to extend a right of first refusal to NSC for the supply of aspartame in 2005 but excluding any trademark license agreements, are hereby canceled and superseded. This Agreement may not be modified except in writing signed by both parties.
10. Definitions. The term "Affiliate" of a person or entity, as used herein or in the Exhibits hereto, shall mean as to such person or entity, (a) any other person or entity directly, or indirectly through one or more intermediaries, controlling, controlled by, or under common control with such person or entity, (b) any officer, director, partner, member, employee, or direct or indirect beneficial owner of 10% or
3
more of the equity or voting interests of such person or entity, or (c) any subsidiary of any such person or entity; and, in the case of NSC, the term "Affiliate" shall include NutraSweet A.G., a Swiss corporation, and Euro-Aspartame, S.A., a French corporation.
11. Successors and Assigns. This Agreement and the rights and obligations hereunder may not be assigned, provided that this Agreement may be assigned to, and the rights and obligations hereunder shall be binding upon and inure to the benefit of, (i) either party's legal successors and assigns through a reorganization, merger, business combination or similar transaction, or (ii) the acquiror of all or substantially all of the stock of either party or any material portion of the stock or assets of the Buyer's Business or NSC's sweetener ingredients business.
* * * * *
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed as of the sate first written above.
| THE NUTRASWEET COMPANY | MERISANT COMPANY | |||||
By: | /s/ CRAIG R. PETRAY | By: | /s/ ETIENNE J. VEBER | |||
| Name: | Craig R. Petray | Name: | Etienne J. Veber | |||
| Title: | President | Title: | President, CEO | |||
MERISANT COMPANY 1 SARL | ||||||
By: | /s/ ETIENNE J. VEBER | |||||
| Name: | Etienne J. Veber | |||||
| Title: | President, CEO | |||||
4
EXHIBIT A—Powder Product Specifications
See Attached.
5
Quality Specifications
| TITLE: | ASPARTAME, FCC—Raw | |||||||
DOCUMENT NO.: | SUPERSEDES: | REVISION LEVEL: | CHANGE REFERENCE NO.: | EFFECTIVE DATE: | ||||
10039854 | 03/23/2000 | A | 875-00-3543 | 04/25/2002 | ||||
| 1.0 | DESCRIPTION | |||||||
1.1 | Color: | White | ||||||
1.2 | Form: | Powder | ||||||
1.3 | Taste: | Sweet | ||||||
1.4 | Odor: | Characteristic, odorless | ||||||
1.5 | Clarity in Solution: | Clear | ||||||
1.6 | Molecular Formula | C14H18N2O5 | ||||||
Molecular Weight | 294.3 | |||||||
Molecular Structure | 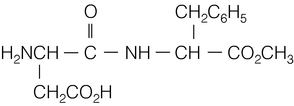 | |||||||
A | 1.7 | STATUS | Kosher Certified | |||||
2.0 | CHEMICAL TESTS | ACCEPTANCE CRITERIA | METHOD | |||||
2.1 | Assay (dried basis) | 98.0-102.0% | CM001 | |||||
2.2 | Diketopiperazine (DKP) | NMT 1.5% | CM001 | |||||
2.3 | Heavy Metals | Not More Than 10 ppm | CM004 | |||||
2.4 | Loss on Drying | Not More Than 4.0% | CM054 | |||||
2.5 | Transmittance | Not Less Than 95.0% | CM114 | |||||
2.6 | Residue on Ignition | Not More Than 0.20% | CM003 | |||||
3.0 | MICROBIOLOGICAL TESTS | ACCEPTANCE CRITERIA | METHOD | |||||
3.1 | Total Aerobic Bacteria | Not More Than 250 cfu per gram | MM001 | |||||
3.2 | Escherichia Coli | None Detectable | MM005 | |||||
3.3 | Salmonella | None Detectable | MM005 | |||||
3.4 | Total Fungal Count | Not More Than 100 cfu per gram | MM001 | |||||
3.5 | Total Coliform Count | Not More Than 10 cfu per gram | MM001 | |||||
4.0 | PHYSICAL TESTS | ACCEPTANCE CRITERIA | METHOD | |||||
4.1 | Color and Form | Conforms to Description | PM002 | |||||
4.2 | Odor | Conforms to Description | PM002 | |||||
4.3 | Taste | Conforms to Description | PM002 | |||||
4.4 | Particle Size | % retained on 100 mesh Not More Than ***% | PM031 | |||||
4.5 | Density | 0.15—0.20 g/mL (Loose) | PM057 | |||||
4.6 | Specific Rotation (20°) | +14.5° to +16.5° | PM037 | |||||
4.7 | Color | LT 1.75 YI | PM079 | |||||
6
5.0 | SAMPLING | |||||||
Refer to SM101 | ||||||||
6.0 | STORAGE | |||||||
6.1 | Preserve in well-closed containers | |||||||
6.2 | Storage areas must meet food regulation sanitation conditions for dry food products. | |||||||
7.0 | VENDOR SHIPPING UNIT AND DOCUMENT MARKING | |||||||
7.1 | NCPI Specification (Component Part) Number. | |||||||
7.2 | NCPI Purchase order Number. | |||||||
7.3 | Vendor Name and Address. | |||||||
7.4 | Vendor Production Lot(s). | |||||||
7.5 | Manufacturer's Name and Manufacturing site (If different from vendor). | |||||||
7.6 | Quantity. | |||||||
7.7 | Description. | |||||||
"Approval signatures on file electronically at Merisant Company Quality Assurance Headquarters"
Julie M. Williams/Merisant; James L. Pumphrey/Merisant
—oOo-
7
EXHIBIT B—FINE GRANULAR PRODUCT SPECIFICATIONS
See Attached.
8
| | | | | | ||||
|---|---|---|---|---|---|---|---|---|
| The NutraSweet Company | Sales Specification | |||||||
TITLE: | ||||||||
| ASPARTAME, GRANULAR | ||||||||
| DOCUMENT NUMBER: | REVISION LEVEL: | MOC NUMBER: | EFFECTIVE DATE: | MATERIAL: | ||||
AE-SL-10-001 | B | 18-1645 | 01-June-2000 | Granular | ||||
(L-Aspartyl L-Phenylalanine Methyl Ester) | ||||||||
| | | | | |||
|---|---|---|---|---|---|---|
| DESCRIPTION | ||||||
CAS | [22839-47-0] | |||||
MOLECULAR WEIGHT | 294.3 | |||||
FORM | Granular | |||||
MOLECULAR FORMULA | C14H18N2O5 | |||||
COLOR | White | |||||
ODOR | Odorless | |||||
TASTE | Sweet | |||||
SOLUBILITY | Sparingly soluble in water; slightly soluble in alcohol | |||||
9
| | | | | |||
|---|---|---|---|---|---|---|
| TEST | ACCEPTANCE CRITERIA | REQUIRED BY | ||||
1. | IDENTITY | |||||
Identification Test (Infrared or HPLC) | Conforms to Standard | FCC, NF, EU | ||||
2. | STRENGTH | |||||
Assay (dried basis) | 98.0% to 102.0% | FCC, NF, EU | ||||
3. | QUALITY | |||||
pH (0.8% solution) | Between 4.5 and 6.0 | FCC, EU | ||||
| Specific Rotation @ 20°C | +14.5° to +16.5° | FCC, NF, EU | ||||
4. | PURITY | |||||
Arsenic (as As) | NMT 3 ppm | EU | ||||
| Diketopiperazine | NMT 1.5% | FCC, NF, EU | ||||
| Heavy Metals (as Pb) | NMT 10 ppm | FCC, NF, EU | ||||
| Loss on Drying | NMT 4.5% | FCC, NF, EU | ||||
| Residue on ignition | NMT 0.2% | FCC, NF, EU | ||||
| Lead | NMT 1 ppm | EU | ||||
| Transmittance | NLT 95.0% | NF, EU | ||||
| Other Related Substances | NMT 2.0% | FCC | ||||
| OR | ||||||
| Chromatographic Impurities | NF | |||||
| Organic Volatile Impurities | Passes | NF | ||||
5. | MICROBIOLOGICAL PROFILE | |||||
Total Aerobic Bacteria | NMT 250 per 1.0 gram | NKC | ||||
| Fungi | NMT 100 per 1.0 gram | NKC | ||||
| Microorganisms of Public Health Concern | None | NKC | ||||
6. | PHYSICAL PROPERTIES | |||||
Particle size distribution | ||||||
| % Retained on 20 mesh | NMT 3.0% | NKC | ||||
| % Retained on 60 mesh | 80—100% | NKC | ||||
| % Through 80 mesh | NMT 3.5% | NKC | ||||
| Unpacked Density | 0.60+/-0.10 g/MI | NKC | ||||
7. | PACKAGING | |||||
Refer to Bill of Material for specific packaging requirements. | ||||||
8. | STORAGE | |||||
Avoid high heat and store under dry conditions. | ||||||
9. | STABILITY/RE-EVALUATION | |||||
| Five years at ambient room temperature storage conditions (typically 15°C to 30°C; 35% to 60% relative humidity). Product should be re-evaluated after this period. | ||||||
APPROVED BY: | DATE: | |||||||
| QA PROJECT LEADER |
10
EXHIBIT C—OTHER TERMS AND CONDITIONS
OTHER TERMS AND CONDITIONS
- 1.
- TERMS. The sale of the product or services described in the contract or invoice of which these terms and conditions are a part (or are on the face hereof) ("PRODUCT") by The NutraSweet Company or its Affiliates ("SELLER") to the buyer identified in the attached contract or invoice ("BUYER") is governed by the following terms and conditions. Seller expressly rejects any additional or different terms or conditions proposed by Buyer.
- 2.
- FORCE MAJEURE. Either party's failure to perform its obligations hereunder (except to make payments hereunder) shall be excused to the extent and for the period of time such nonperformance is caused by an event of force majeure, including but not limited to war, invasion, fire, explosion, food, riot, strikes, acts of God, delays or defaults of carriers, energy shortage, inability to obtain raw materials, acts of government, its agencies or instrumentalities, or contingencies or causes beyond such party's reasonable control.
- 3.
- PRICE, All prices are *** nearest *** in ***, *** and *** and *** in ***. For avoidance of doubt, *** shall pay ***. Seller will use commercially reasonable efforts to have shipments made in accordance with any timely instructions of Buyer or estimated dates provided in writing to Seller. Title to, and risk of loss of, any shipment hereunder will *** at ***.
- 4.
- PAYMENT AND TERMINATION. Unless otherwise stated in writing by the Seller, the price for the Product is payable *** and payment is due to Seller within *** of the date of invoice. Any amounts not paid when due will have interest from the date due until paid at an annual rate equal to ***% *** in effect at *** on the date such payment was due. Seller reserves the right, among other remedies, either to *** or to *** under it in the event Buyer *** for *** after notice of such *** and failure to cure within *** of receipt of such notice. Should *** become unsatisfactory to ***, *** or *** satisfactory to *** may be required by *** for *** and for ***.
- 5.
- WARRANTIES BY SELLER. Seller hereby warrants to Buyer with respect to the Product that:
- (a)
- It has good and marketable title to the Product shipped to Buyer hereunder;
- (b)
- At the time of shipment to Buyer, the Product will meet Seller's then current specifications; and
- (c)
- For Product sold as a food ingredients product, at the time of shipment to Buyer, the Product will not be adulterated or misbranded within the meaning of the United States Food, Drug and Cosmetic Act or any of the regulations thereunder.
THE WARRANTIES SET FORTH IN THIS SECTION 5 ARE IN LIEU OF ANY AND ALL OTHER WARRANTIES, REPRESENTATIONS OR CONDITIONS, EXPRESS OR IMPLIED, COLLATERAL, STATUTORY OR OTHERWISE, AND WHETHER IN CONTRACT, TORT OR OTHERWISE SALE OF THE PRODUCT IS MADE ON THE UNDERSTANDING THAT THERE ARE NO EXPRESS OR IMPLIED WARRANTIES THAT THE PRODUCT DELIVERED HEREUNDER WILL BE MERCHANTABLE OR FIT FOR ANY PARTICULAR PURPOSE.
- 6.
- LIMITATION ON CLAIMS. All claims that any shipments hereunder does not conform to the above warranties will be waived by Buyer with respect to such shipment unless written notice is given to Seller by Buyer accompanied by a sample of the alleged non-conforming Product within *** after Buyer's receipt of the shipment. Buyer shall not conduct any post sales audit of compliance with any terms and condition of sale and hereby waives any claims resulting therefrom unless such audit and claim are completed within two years of the date of the relevant order.
11
- 7.
- REPLACEMENT OR CREDIT BY SELLER. Seller will notify Buyer within 30 days after receipt of Buyer's notice provided pursuant to Section 6 above whether Seller accepts Buyer's claims. If Seller accepts such claim, it will instruct Buyer either to return the shipment or destroy it, and Buyer will promptly comply with such instruction at Seller's expense. Seller will promptly replace any such Product at its own expense on the same shipping terms as the original shipment or issue a credit note to Buyer for such shipment including shipping charges paid by Buyer.
- 8.
- LIMITED REMEDY. Except as provided in Article 10A with respect to third party claims, the exclusive remedy of Buyer arising out of breach of the above warranties will be replacement or credit, at Seller's option.
- 9.
- LIMITATION OF LIABILITY. Seller will not in any event be liable to Buyer, to Buyer's Affiliates, or to Buyer's franchisees, co-packers, or distributors (if any) for special, indirect or consequential damages (including but not limited to lost profits, manufacturing costs, damage to goodwill, or loss of business), or product recall costs whether based on the use of Product or any goods, incorporating Product (whether or not the Product involved conforms to Seller's specifications and warranties set forth herein) or on Seller's late delivery or non-delivery of Product.
- 10.
- INDEMNITY.
- (a)
- IN FAVOR OF BUYER. Seller will indemnify, defend and hold harmless Buyer, its Affiliates and their respective officers, directors, employees, agents and representatives from and against liability, damage, loss, cost or expense (including reasonable attorney's fees and costs) arising out of any third party claims or suits resulting from Seller's negligent act or omission, breach of this Agreement or breach of warranty in the manufacture or sale of Product hereunder.
- (b)
- IN FAVOR OF SELLER. Buyer will indemnify, defend and hold harmless Seller, its Affiliates and their respective officers, directors, employees, agents and representatives from and against any and all liability, damage, loss, cost or expense (including reasonable attorney's fees and costs) of any kind or nature whatsoever arising out of any third party claims or suits resulting from (a) Buyer's negligent act or omission in connection with the purchases, storage, use, sale, shipment, promotion, or distribution of Product sold hereunder or of any goods (including their manufacture and sale) in which Product is incorporated; (b) product liability claims relating to the manufacture, promotion or sale of Buyer's goods incorporating Products; and (c) claims of contributory infringement or inducement of infringement against Seller based on infringement by Buyer of any third party intellectual property right(s) covering Buyer's goods incorporating Product, including all materials or intermediates produced or used in their manufacture (excluding Product) or method(s) for its manufacture or use.
- (c)
- NOTICE OF CLAIM. Promptly after receiving notice of any claim or lawsuit to which this Section 10 applies, the party seeking to be indemnified will notify the other party in writing, and the party so notified will immediately assume responsibility at its sole expense for the handling and defense of such claim or suit on behalf of the party entitled to indemnify. The parties will fully cooperate with each other on such defense.
- 11.
- TAXES. Buyer will pay all sales, revenue, excise or other federal, state, local or foreign taxes (including value added and consumption taxes) and all import or export duties payable with respect to any shipment hereunder, excluding Ad Valorem taxes of Seller and taxes based on Seller's net income.
- 12.
- GOVERNING LAW. The contract or invoice of which these terms and conditions are a part (or are on the face hereof) shall be governed by, and interpreted in accordance with the laws of the State of Illinois, U.S. except any such law mandating the application of the law(s) of a different jurisdiction.
12
- 13.
- LAW VIOLATION. If any provision hereof is or becomes, a violation of any law, rule, order or regulation issued thereunder, Seller shall have the right, upon notice to Buyer, to cancel such provision without effect upon the other provisions, or to cancel further deliveries in their entirety.
- 14.
- INTELLECTUAL PROPERTY.
- (a)
- PATENTS. Seller warrants that, to its knowledge, the sale of the material hereunder will not infringe the claims of any United States Patent covering the material itself, but in the event that it is alleged that such sale constitutes infringement of such patent, then Seller's liability to Buyer shall:
- (i)
- be limited to the defense of such infringement actions and the payment of damages awarded therefor by a court of competent jurisdiction from which no appeal is or can be taken, or the settlement of such actions, as Seller shall elect, and
- (ii)
- arise only if Buyer promptly gives Seller written notice of such claim and full authority, information and assistance for the defense and/or settlement of such claim.
- This section 14(a) states the entire liability of Seller with respect to patent infringements by said materials. Seller does not warrant against infringement by reason of any use of the material or of its combination with any other material or in the operation of any process. Seller reserves the right to suspend deliveries hereunder, or to terminate this contract, if Seller believes that the manufacture and/or sale by Seller, or the use by Buyer, of any material sold hereunder infringes any Patent.
- (b)
- TRADEMARK USAGE. Buyer agrees that, if Seller grants Buyer any right to use any Seller trademark, unless as otherwise provided in any separate agreement between Buyer and Seller, its use of the Seller trademarks and the advertising and packaging of Buyer's goods will be in accordance with Seller's policies and procedures with respect to the use of any of Seller's trademarks as provided to Buyer from time to time. Buyer will not grant rights of any kind of the Seller trademarks to any third party.
- 15.
- NO RIGHT OF SET-OFF. Buyer waives any right it now has or later acquires to set off any amount due from Seller or its affiliates against amounts owed by Buyer hereunder.
- 16.
- ALLOCATION. If Seller determines that its ability to supply the total demand for the product, or obtain any or a sufficient quantity of any material used directly, or indirectly in the manufacture of the Product, is hindered, limited or made impracticable, Seller may allocate its available supply of the Product or such material (without obligation to acquire other supplies or any such product or material) among itself and its customers on such basis as Seller determines to be equitable and without liability for any failure of performance which may result therefrom.
- 17.
- ASSIGNMENT. The terms, conditions and obligations of this Agreement will inure to the benefit of and be binding upon the parties hereto and the respective successors and assigns thereof. This Agreement and the rights and obligations hereunder may not be assigned, provided that this Agreement may be assigned to, and the rights and obligations hereunder shall be binding upon and inure to the benefit of, (i) either party's legal successors and assigns through a reorganization, merger, business combination or similar transaction, or (ii) the acquiror of all or substantially all of the stock of either party or any material portion of the stock or assets of the Buyer's Business or NSC's sweetener ingredients business.
- 18.
- SEVERABILITY. The provisions contained herein are severable and the contract or invoice of which these terms and conditions are a part (or are on the face hereof() shall be interpreted as if all completely invalid or unenforceable provisions were not contained herein and partially valid and enforceable provisions shall be enforced to the extent valid and enforceable. If any applicable and binding law or rule of any jurisdiction renders any provision of the contract or invoice of which these terms and conditions are a part (or are on the face hereof) unenforceable, the parties
13
hereto agree to modify, or any modification made or ordered by any court, arbitrator, or governmental agency of, such invalid or unenforceable provision, to the extent required to be valid and enforceable in such jurisdiction. Such modifications to this Agreement shall be effective only in such jurisdiction and the contract or invoice of which these terms and conditions are a part (or are on the face hereof) shall be enforced as originally made and entered into in all other jurisdictions.
14
EXHIBIT D—PRICING
| | | | ||
|---|---|---|---|---|
| 1. | During calendar year 2004, volume, price and Territory for Product will be as follow: | |||
Volume: | *** of Powder Product | |||
Price: | $*** per *** for Powder Product | |||
Territory: | *** | |||
2. | During calendar year 2005, volume, price and Territory for the Product will be as follows: | |||
Volume: | *** of Product | |||
Price | $*** per *** for Powder Product | |||
$*** per *** for Fine Granular Product | ||||
Territory: | *** | |||
CONFIDENTIAL MATERIAL APPEARING IN THIS DOCUMENT WAS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION IN ACCORDANCE WITH RULE 24b-2, PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. OMITTED INFORMATION WAS REPLACED WITH ASTERISKS.
15