Sample Business Contracts
License Agreement - Arizona Board of Regents and Seattle Genetics Inc.
Free Customizable Licensing Forms
- Copyright License Agreement
- End User License Agreement
- License Agreement
- Manufacturing License Agreement
- Master Use License Agreement
- Software License Agreement
- Trademark License Agreement
- Domain Name Assignment
- Invention Assignment Agreement
- Patent Application Assignment
- Patent Assignment
- Trademark Assignment
Sponsored Links
LICENSE AGREEMENT
No. 651-O1.LIC
THIS AGREEMENT (the "Agreement"), made this 3rd day of February, 2000 (the "EFFECTIVE DATE"), is by and between the ARIZONA BOARD OF REGENTS, a body corporate of the State of Arizona, acting on behalf of and for ARIZONA STATE UNIVERSITY, of Tempe, Arizona ("ASU") and Seattle Genetics, Inc., a corporation organized under the laws of Delaware having its principal place of business located at 22215 26th Avenue SE, Bothell, Washington 98021 ("LICENSEE").
RECITALS
| A. | A certain invention, generally characterized as a drug known under the name "Auristatin E", hereinafter referred to as the "TECHNOLOGY", was made in the course of research at ASU by G. Robert Pettit and Jozsef Barkoczy and are and will be covered by ASU's PATENT RIGHTS, as defined below. |
| B. | The National Cancer Institute sponsored part of the development of the TECHNOLOGY (as defined below) and as a consequence this license is subject to overriding obligations to the Federal Government as set forth in 35 U.S.C. 200-212 and applicable governmental implementing regulations. |
| C. | The LICENSEE is a "small business firm" as defined in 15 U.S.C. 632. |
| D. | ASU represents and warrants that it has the right to grant licenses to make, have made, use and sell products or services covered by ASU's PATENT RIGHTS under such patent rights, together with any patents which have issued and which may yet issue on it. |
| E. | ASU is desirous that the TECHNOLOGY be developed and utilized to the fullest extent so that the benefits can be enjoyed by the general public; and |
| F. | The LICENSEE is desirous of obtaining certain rights from ASU for the commercial development, use, and sale of products or services covered by ASU's PATENT RIGHTS, and ASU is willing to grant such rights on the terms and conditions set forth herein. |
AGREEMENT
DEFINITIONS
| 1.1. | "AFFILIATE" means any corporation or other business entity that, directly or indirectly, controls, is controlled by or is under control with LICENSEE where control shall mean: (a) in the case of corporate entities, direct or indirect ownership of at least fifty percent (50%) of the stock or participating shares entitled to vote for the election of directors; and (b) in the case of non-corporate entities, direct or indirect ownership of at least fifty percent (50%) of the equity interest or the power to direct the management and policies of such entity; provided, however, that in any country where the local law shall not permit foreign equity participation of at least 50%, then an "AFFILIATE" shall include any company in which the LICENSEE shall own or control, directly or indirectly, the maximum percentage of such outstanding stock or voting rights permitted by local law. |
| 1.2. | "ASU's PATENT RIGHTS" shall mean patent rights to certain subject matter, which is included in the following: |
Under ASU Case No. 651:
U.S. Patent No. 5,635,483 entitled "Tumor Inhibiting Tetrapeptide Bearing
Modified Phenethyl Amides".
For the purposes of this Agreement, only those compounds taught in the above named patent and identified as stereoisomers of a compound commonly referred to as "Auristatin E" are included in this license and the grant of rights described in Article 2 of this Agreement shall pertain only to the following:
Auristatin E, Compound No. 1S2R
Auristatin E, Compound No. 1R2R
Auristatin E, Compound No. 1S2S
Auristatin E, Compound No. 1R2S
Each of which falls within the general structure shown below:
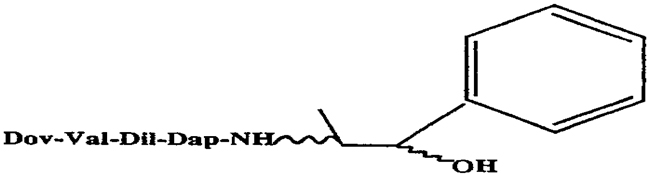
-2-
and any corresponding extensions or foreign applications or patents.
| 1.3. | "INVENTOR" shall, for the purposes of the Agreement, mean Dr. George R. Pettit and Jozsef Barkoczy whose names appear on the patent described by ASU's PATENT RIGHTS. |
| 1.4. | "KNOW-HOW" shall mean all technical data, information, materials and technical expertise that relates to TECHNOLOGY, including without limitation, chemical and physical data and techniques, clinical data, medical uses, product forms, formulations, and specifications. |
| 1.5. | "LICENSED FIELD OF USE" shall mean all uses of LICENSED PRODUCTS or LICENSED METHODS. |
| 1.6. | "LICENSED METHOD" shall mean any method, procedure, process or other subject matter whose use or practice would constitute, but for any license granted to LICENSEE hereunder, an infringement of any VALID CLAIM contained in ASU's PATENT RIGHTS, as defined herein. |
| 1.7. | "LICENSED PRODUCT' shall mean any material, composition, composition of matter, compound, device or embodiment the manufacture, use or sale of which would constitute, but for the license granted to the LICENSEE pursuant to this Agreement, an infringement of any VALID CLAIM contained in ASU's PATENT RIGHTS, as defined herein. For the purposes of this Agreement, LICENSED PRODUCT shall include combinations of chemical compounds in which a single agent such as Auristatin E is combined with another compound such as an antibody. |
| 1.8. | "NET SALES" means the total of the gross invoice prices of LICENSED PRODUCTS sold by the LICENSEE, an AFFILIATE, or a SUB-LICENSEE, less the sum of the following actual and customary deductions where applicable: cash, trade, or quantity discounts; sales, use, tariff, import/export duties or other excise taxes imposed upon or levied with respect to any sale of LICENSED PRODUCTS; transportation and insurance charges; and allowances or credits because of rejections or returns or uncollectible amounts. Transfers to an AFFILIATE or SUB-LICENSEE for end use by the AFFILIATE or SUB-LICENSEE shall be treated as NET SALES. |
| 1.9. | "SUB-LICENSEE" shall mean any corporation or other business entity to which the LICENSEE has granted a sub-license under the ASU's PATENT RIGHTS, as permitted herein. |
| 1.10. | "TECHNOLOGY" shall mean certain inventions relating to Auristatin E, which were made in the course of research at ASU by Drs. G. Robert Pettit, and Jozsef Barkoczy which are covered by ASU's PATENT RIGHTS, as defined herein. |
| 1.11. |
"TERM" shall mean the period of time commencing on the EFFECTIVE DATE and continuing until the expiration in each country of the last to expire patent contained in |
-3-
|
ASU's PATENT RIGHTS. |
| 1.12. | "TERRITORY" shall mean world-wide. |
| 1.13. | "VALID CLAIM" shall mean a claim in any issued unexpired United States patent and a pending claim in any corresponding foreign patent application included in ASU's PATENT RIGHTS which has not been held unpatentable or invalid or unenforceable by a decision of a court or other competent authority, which is not appealable or not appealed within the time allowed for appeal, and which has not been admitted to be invalid through reissue or disclaimer or otherwise and which is not subject to an interference claim. |
2. GRANT
| 2.1. | ASU hereby grants to LICENSEE an exclusive license in the TERRITORY and in the LICENSED FIELD OF USE, which shall include the right to grant sub-licenses, under ASU's PATENT RIGHTS, as specified in Paragraph 1.1, to develop, have developed, make, have made, market, import, sell, and otherwise use LICENSED PRODUCTS and to practice the LICENSED METHODS under ASU's PATENT RIGHTS. |
| 2.2. | Except as otherwise provided herein, the rights granted in Paragraph 2.1 shall extend for the TERM of this Agreement. |
| 2.3. | The license granted hereunder may be subject to all the applicable provisions of any Licenses to the United States Government executed by ASU. The license granted hereunder is subject to the overriding obligations to the U.S. Government set forth in 35 U.S.C. 200-2 12 and applicable governmental implementing regulations. |
| 2.4. | ASU expressly reserves the right to use the TECHNOLOGY for educational and non-commercial research purposes. |
| 2.5. | This Agreement and grant of license hereunder are subject to the terms and conditions contained throughout this agreement including the Licensee's specific diligence obligations specified in Article 8. |
3. SUB-LICENSES
| 3.1. | ASU also hereby grants to the LICENSEE the right to issue sub-licenses in the TERRITORY to SUB-LICENSEES to develop, have developed, make, have made, market, import, sell and otherwise use LICENSED PRODUCTS and to practice the LICENSED METHODS, provided the LICENSEE has current rights thereto under this Agreement. To the extent applicable, such sub-licenses shall include all of the rights of and obligations due to ASU (and, if applicable, the United States Government) that are contained in this Agreement including without limitation those obligations set forth in Article 25. |
| 3.2. |
The LICENSEE shall provide ASU with a copy of each sub-license issued within thirty |
-4-
|
(30) days after execution; collect and guarantee payment of all royalties due ASU from SUB-LICENSEES; and deliver all reports due ASU from SUB-LICENSEES, as provided herein. |
| 3.3. | Except as otherwise provided in Paragraph 16.4 hereof, upon termination of this Agreement for any reason, ASU, at its sole discretion, may determine whether any or all sub-licenses are to be canceled or assigned to ASU. |
4. LICENSE ISSUE FEE AND MILESTONE PAYMENTS
| The | LICENSEE agrees to pay to ASU a non-refundable Issue Fee of $20,000.00 within 10 days of the EFFECTIVE DATE. |
5. PAYMENTS AND ROYALTIES
| 5.1. | License Maintenance Payments: |
| 5.1.1. | The LICENSEE shall pay to ASU an annual maintenance fee beginning at $30,000.00 and increasing by annual increments of $5,000.00 each year up to a maximum of $50,000.00 per year. For example, the first annual maintenance fee will be $30,000.00; the second will be $35,000.00; the third will be $40,000.00; the fourth will be $45,000.00 and the fifth will be $50,000.00 and will continue each year thereafter at the $50,000.00 rate. |
| 5.1.2. | Such annual maintenance fee payments shall be due and payable each year until a "New Drug Approval" (NDA) is received by the US Food and Drug Administration. No maintenance fee shall be due during the years following receipt of the NDA. |
| 5.1.3. | The first such annual maintenance fee payment shall be due and payable on the first year anniversary of the EFFECTIVE DATE. |
| 5.1.4. | Commencing with the second annual maintenance fee, the annual maintenance fee shall be payable in two equal installments. The first installment shall be due and payable on the six-month anniversary of the EFFECTIVE DATE and the second installment shall be due and payable on the one-year anniversary of the EFFECTIVE DATE. |
| 5.2 | Milestone Payments |
| 5.2.1. | The LICENSEE shall make milestone payments to ASU upon the achievement of certain clinical development milestones according to the schedule, below: |
| 5.2.2. | Upon initiation of the first Phase III clinical study in which the LICENSED PRODUCT is a single agent such as Auristatin E: |
$100,000.00
-5-
| 5.2.3. | Upon initiation of the first Phase III clinical study in which the LICENSED PRODUCT that constitutes a combination of a single agent such as Auristatin E and another compound such as an antibody: |
$100,000.00
| 5.2.4. | Upon FDA approval of an NDA for a LICENSED PRODUCT that is a single agent such as Auristatin E: |
$250,000.00
| 5.2.5. | Upon FDA approval of an NDA for a LICENSED PRODUCT that constitutes a combination of a single agent such as Auristatin E and another compound such as an antibody: |
$250,000.00
| 5.3. | Earned Royalties: |
| 5.3.1. | The LICENSEE shall pay to ASU an EARNED ROYALTY of 5.5% of the NET SALES of all LICENSED PRODUCTS that utilize a process or composition of matter covered by claims contained in ASU's PATENT RIGHTS and that constitute a single agent, i.e.: Auristatin E. |
| 5.3.2. | The LICENSEE shall pay to ASU an EARNED ROYALTY of 2.0% of the NET SALES of all LICENSED PRODUCTS that utilize a process or composition of matter covered by claims contained in ASU's PATENT RIGHTS and that constitute a combination of a single agent such as Auristatin E and another compound such as an antibody. |
| 5.3.3. | The LICENSEE shall pay to ASU an earned royalty ("EARNED ROYALTY") in accordance with the rules specified in Paragraphs 5.4 through 5.14. LICENSEE's and/or SUB-LICENSEE's obligation to pay EARNED ROYALTIES shall commence with the first sale of any LICENSED PRODUCT and will continue as long as the LICENSEE and/or SUB-LICENSEES are selling any LICENSED PRODUCT throughout the TERM and in accordance with the sub-paragraphs, below: |
| 5.4. | Minimum Annual Royalty: |
The LICENSEE shall pay to ASU a minimum annual royalty of $50,000 for the life of VALID CLAIMS of ASU's PATENT RIGHTS (the "Minimum Annual Royalty"), beginning in the year of LICENSEE's first receipt of marketing approval for a LICENSED PRODUCT from the US Food and Drug Administration ("US FDA") or any other analogous worldwide regulatory agency. LICENSEE shall account to ASU and pay royalties to ASU semi-annually within forty-five (45) days after the end of each calendar half-year for the just preceding calendar half-year. Concurrent with the final royalty payment, due on February 15 following any year, LICENSEE shall make an additional payment if necessary in order to meet its obligation to make minimum royalty payments
-6-
for that year.
| 5.5. | For sales of LICENSED PRODUCTS to an AFFILIATE of LICENSEE at a reduced price from that customarily charged to an unrelated third party, the royalty paid to ASU shall be based on the NET SALES of such LICENSED PRODUCTS by the AFFILIATE to the AFFILIATE'S customers. Where the LICENSEE sells LICENSED PRODUCTS for end use to itself or an AFFILIATE, such sale shall be considered a sale at list price, and ASU shall be entitled to receive a royalty in accordance with this Article based on such list price. Each reference to the LICENSEE shall be meant to include its AFFILIATES. |
| 5.6. | In the event LICENSEE sells LICENSED PRODUCTS to a SUB-LICENSEE for end use by that SUB-LICENSEE, such sale shall be considered a sale at list price, and ASU shall be entitled to receive a royalty in accordance with this Article based on such list price. If no such list price is available, such sale shall be considered a sale at a commercially reasonable price between arm's length parties, as determined in good faith by LICENSEE and based on its sales price to other arm's length third parties, if available. |
| 5.7. | Article 1 defines A5U's PATENT RIGHTS and LICENSED PRODUCTS so that royalties are payable on LICENSED PRODUCTS covered by a Valid Claim. EARNED ROYALTIES shall be due on LICENSED PRODUCTS in each country where relevant ASU's PATENT RIGHTS exist, for the duration of VALID CLAIMS of such ASU'S PATENT RIGHTS in such country. EARNED ROYALTIES shall accrue to ASU when LICENSED PRODUCTS are invoiced, or if not invoiced, when delivered to a third party and shall be paid as set forth below. |
| 5.8. | Commencing upon the first commercial sale of a LICENSED PRODUCT, the LICENSEE shall pay EARNED ROYALTIES accruing to ASU on a semi-annual basis on or before the following dates of each calendar year: |
• February 15
• August 15
| 5.9. | Each payment pursuant to this Article 5 shall be for EARNED ROYALTIES that accrued within the LICENSEE's most recently completed calendar half-year and shall be accompanied by a royalty report. Such reports shall indicate for the relevant calendar half-year the NET SALES of the LICENSED PRODUCT manufactured or sold by LICENSEE, its AFFILIATES and its SUB-LICENSEES with respect to which payments are due, and the amount of those payments. If no payment is due for any period, LICENSEE shall so report. |
| 5.10. | All moneys due ASU shall be payable in United States funds collectible in Tempe, Arizona. When LICENSED PRODUCTS are sold for moneys other than United States dollars, the EARNED ROYALTIES shall first be determined in the foreign currency of the country in which such LICENSED PRODUCTS were sold and then converted into equivalent United States funds. The exchange rate for such conversion shall be that rate quoted in The Wall Street Journal on the last business day of the reporting period. |
-7-
| 5.11. | Royalties earned with respect to sales occurring in any country outside the United States shall not be reduced by any taxes, fees, or other charges imposed by the government of such country on the remittance of royalty income. Notwithstanding the foregoing, all payments made by the LICENSEE in fulfillment of ASU's tax liability in any particular country shall be credited against Earned Royalties or fees due ASU for that country and shall be reported to ASU along with payment of EARNED ROYALTIES, net of any such amount. |
| 5.12. | If at any time legal restrictions prevent the prompt remittance of part or all royalties by the LICENSEE with respect to any country where a LICENSED PRODUCT is sold, royalty payments attributable to sales in that country shall be suspended for as long as such prohibition is in effect and as soon as such prohibition ceases to be in effect, all royalties that ASU would have been entitled to but for the prohibition shall be deposited or transmitted to the extent allowable. |
| 5.13. | If any patent or any claim included within ASU's PATENT RIGHTS is held invalid in a final decision by a court of competent jurisdiction and last resort and from which no appeal has or can be taken, all obligation to pay royalties hereunder based on such patent or claim or any patentably indistinct claim shall cease as of the date of such final decision. Royalty payments shall be suspended during any period in which the PATENT RIGHTS are subject to any interference or other proceeding disputing the validity of the PATENT RIGHTS. Once such interference or other proceeding is concluded and such disputed claims within ASU's PATENT RIGHTS are upheld, LICENSEE shall make a royalty payment to ASU to cover the royalty due on all NET SALES during the suspension and continuing royalty payments and obligations to ASU shall be resumed as though no interference or other proceeding ever took place. |
| 5.14. | No royalties shall be collected or paid on LICENSED PRODUCTS sold to the account of the U.S. Government, any agency thereof, or any state or domestic municipal government as provided for in any License to the Government. |
6. PATENT EXPENSES and PATENT PROSECUTION AND MAINTENANCE
| 6.1. | The costs of preparing, filing, prosecuting and maintaining all United States and foreign patent applications and issued patents from and after the EFFECTIVE DATE shall be borne by ASU. These costs include any patent prosecution costs that are incurred for appeals, re-examination, re-issue, interferences, or inventorship determinations as well as the maintenance of all resulting patents. ASU shall prepare and deliver to LICENSEE a report setting forth the countries in which it has filed and intends to file applications with respect to ASU's PATENT RIGHTS. LICENSEE may request ASU in writing to file applications in additional countries. LICENSEE shall be responsible for any incremental costs incurred by ASU for any countries added in accordance with the foregoing after ASU's receipt of such request from LICENSEE. |
| 6.2. | All patents comprising or including ASU's PATENT RIGHTS shall be held in the name |
-8-
|
of ASU and shall be obtained and maintained using counsel who are on ASU's list of officially approved intellectual property counsel, and who are approved by LICENSEE, such approval not to be unreasonably withheld. |
| 6.3. | ASU shall use reasonable efforts to obtain, file, prosecute and maintain the United States and foreign patents comprising ASU's PATENT RIGHTS at ASU's sole discretion. ASU agrees to consult with LICENSEE and give good faith consideration to LICENSEE's comments regarding such patent prosecution and related proceedings to the extent related to the TECHNOLOGY. It is understood by LICENSEE that ASU counsel takes instructions only from ASU. |
| 6.4. | The LICENSEE shall have the right to request that ASU obtain patent protection on the TECHNOLOGY in foreign countries if available and if it so desires. The LICENSEE shall notify ASU within seven (7) months of the filing of the corresponding United States application of its decision to obtain additional foreign patents. This notice concerning foreign filing shall be in writing, shall identify the countries desired, and shall reaffirm the LICENSEE's obligation to underwrite the incremental costs thereof. |
| 6.5. | ASU shall use all reasonable efforts to amend any patent application to include claims reasonably requested by the LICENSEE to protect the products contemplated to be sold under this Agreement, or to cover competitive products as long as such amendments, in the opinion of ASU's patent counsel will not jeopardize issuance of a patent. ASU shall pursue all claims of improper inventorship regarding any patent or patent application, which is or would be subject to the licenses granted hereunder as reasonably requested by LICENSEE and at LICENSEE's expense. |
| 6.6. | ASU's obligation to prosecute any patent application shall cease at such time when ASU is advised by counsel that such patent application has been rejected and an appropriate appeal procedure must be pursued in order to gain issuance of the patent; or if an order for reexamination is issued by the patent office; or if an interference is declared; or if a patent reissuance is required or requested by the LICENSEE. If, however, upon notification by ASU, LICENSEE re-affirms its obligation in writing that LICENSEE will reimburse ASU for the costs involved in such appeals process, ASU will proceed with the necessary action. |
| 6.7. | ASU shall promptly advise a LICENSEE of the grant, lapse, revocation, surrender or invalidation of any ASU PATENT RIGHT. ASU shall not abandon or irrevocably limit the scope of any ASU PATENT RIGHT in any country without the prior written consent of LICENSEE. |
7. TECHNICAL INFORMATION and DUE DILIGENCE
| 7.1. | Within 60 days of the EFFECTIVE DATE, the ASU Cancer Research Institute shall supply one-hundred milligrams (100 mg.) of Auristatin E to the LICENSEE. |
-9-
| 7.2. | Within 60 days of the EFFECTIVE DATE, ASU shall transfer to LICENSEE all KNOW-HOW, preclinical data, assays and associated materials, protocols, procedures and any other information in ASU's possession or control, necessary or desirable to develop the TECHNOLOGY. |
| 7.3. | Each party, for itself and any SUB-LICENSEE'S, undertakes during the TERM of this Agreement, and for a period of 5 years following the termination of this Agreement, to hold in confidence and not to use or disclose to any third party, except as permitted herein, the TECHNOLOGY and KNOW-HOW received from the other party. This obligation shall not apply to the portion(s) of TECHNOLOGY and KNOW-HOW which: |
| 7.3.1. | was known to the receiving party or any of its SUB-LICENSEES prior to its receipt by the receiving party, and can be so proved by written or electronic records; or |
| 7.3.2. | is received at any time by the receiving party or its SUB-LICENSEES in good faith from a third party lawfully in possession of it and having the right to disclose the same, and can be so proved by written or electronic records; or |
| 7.3.3. | is as of the date of receipt by the receiving party in the public domain or subsequently enters the public domain other than by reason of acts or omissions of the employees or agents of the receiving party or its SUB-LICENSEES, and can be so proven by written or electronic records; or is independently developed by the receiving party without any reference to the TECHNOLOGY or KNOW-HOW, and can be so proved by written or electronic records. |
| 7.4. | LICENSEE and its SUB-LICENSEES may use and discuss the TECHNOLOGY and KNOW-HOW received from ASU in connection with applying for and securing necessary governmental authorization for the lawful marketing of LICENSED PRODUCTS in the TERRiTORY; and in connection with LICENSEE's financing activities. |
| 7.5. | Notwithstanding the provisions of Paragraph 7.3, ASU reserves the right to publish information of scientific importance, including any TECHNOLOGY and KNOW-HOW; provided, however, that (1) the TECHNOLOGY and KNOW-HOW or the material part of it shall, prior to such publication, have been made the subject of a United States patent application, or (2) LICENSEE, upon review as provided for herein, shall have declined to comment within the prescribed period of time. ASU shall furnish LICENSEE with a copy of every relevant publication by ASU pursuant to this Article, prior to publication of the information. LICENSEE shall have 30 days from receipt of the intended publication to indicate to ASU any reasonable revisions or deletions it deems necessary to protect its proprietary rights. Title to any copyrightable material, first produced or composed by one party, shall remain with that party; provided an irrevocable, royalty-free right to reproduce, translate and use the copyrighted material for purposes of this Agreement shall be granted to the other party. |
8. DUE DILIGENCE AND MARKETING OBLIGATIONS
| 8.1. | The LICENSEE, upon execution of this Agreement, shall diligently proceed with the |
-10-
|
development, manufacture and sale of LICENSED PRODUCTS and shall diligently endeavor to market the same within a reasonable time after execution of this Agreement and in quantities sufficient to meet the market demands. LICENSEE shall promptly notify ASU in writing of the commencement of such marketing. |
| 8.2. | LICENSEE shall promptly advise ASU in writing if it decides (i) not to market any LICENSED PRODUCT in any country, (ii) to discontinue the marketing of such LICENSED PRODUCT in any country, or (iii) to not resume the marketing of such LICENSED PRODUCT in any country following expiration. This notice shall, unless LICENSEE is (a) developing another LICENSED PRODUCT which is either superior, based on data available at that time, or is at the same or a later stage of development than the abandoned LICENSED PRODUCT or (b) marketing another LICENSED PRODUCT for the same indication in such country at such time, serve to terminate this Agreement as to that LICENSED PRODUCT and that country. |
| 8.3. | The Parties shall promptly advise one another of any confirmed instance which comes to either party's attention of severe or unexpected reactions from the use of TECHNOLOGY or any LICENSED PRODUCT. |
| 8.4. | LICENSEE shall, in a manner consistent with the effort devoted to products of the same or similar potential of its own development, prepare and file or cause to be prepared and filed all necessary applications to obtain approval for LICENSED PRODUCTS in the name of LICENSEE or its AFFILIATES or SUB-LICENSEES from any necessary governmental authorities. |
| 8.5. | LICENSEE shall in the performance of any investigation, testing and solicitation of government approvals pertaining to the use of the TECHNOLOGY, exercise at least the same degree of diligence which any reasonable and prudent manufacturer exercises in the investigation, testing and solicitation of government approvals for an invention of similar class or utility invented by employees of and owned by the manufacturer. |
| 8.6. | The LICENSEE agrees to the following scientific and clinical development requirements. |
| 8.6.1. | Perform in vitro cytotoxicity assays on Auristatin E and complete same no later than September, 2000. |
| 8.6.2. | Attempt preparation of monoclonal antibody-drug conjugates with Auristatin E and complete same no later than September, 2000. |
| 8.6.3. | Perform in vitro cytotoxicity assays on successfully prepared monoclonal antibody-drug conjugates and complete same no later than February, 2001. |
| 8.6.4. | Perform in vivo antitumor experiments on Auristatin E and/or Auristatin E monoclonal antibody-drug conjugates that meet cytotoxicity requirements and complete same no later than September, 2001. |
| 8.6.5. | Perform toxicology experiments on Auristatin E and/or Auristatin E monoclonal antibody-drug conjugates that meet cytotoxocity and efficacy requirements and complete same no later than September, 2002. |
-11-
| 8.6.6. | Scale up production of Auristatin E and/or Auristatin E monoclonal-drug conjugates that meet cytotoxicity, efficacy, and toxicology requirements and complete same no later than September, 2003. |
| 8.6.7. | Prepare and file IND application on Auristatin E or Auristatin E monoclonal antibody-drug conjugates that meet clinical development requirements and complete same no later than February, 2004. |
| 8.6.8. | If an IND Application is approved, initiate and conduct or have conducted at least one Phase I clinical trial no later than May, 2004. |
| 8.7. | LICENSEE shall meet the reasonably anticipated market demand for LICENSED PRODUCTS following commencement of marketing and during the TERM of this Agreement. |
| 8.8. | If LICENSEE fails to meet any of the above requirements as set forth in Paragraphs 8.6.1 through 8.6.8, then ASU shall have the right to notify LICENSEE of ASU's belief in writing that LICENSEE has failed to meet any such specific obligation with respect to any specified LICENSED PRODUCT(S) and to request LICENSEE to undertake immediate remedial action. If the parties disagree as to any such failure, either may bring the matter up for arbitration in Maricopa County, Arizona upon sixty (60) days prior written notice under the then prevailing rules of the American Arbitration Association for adjudication. If, during the arbitration process, it is determined that LICENSEE has not acted diligently with respect to such LICENSED PRODUCT(S), then LICENSEE has the right to undertake remedial action. If the LICENSEE fails to do so, within a time deemed reasonable by the arbitrator, LICENSEE'S rights under this Agreement(s) may be terminated by ASU with respect to the relevant LICENSED PRODUCT(S) pursuant to Paragraph 16.1. |
9. REPORTS
| 9.1. | Beginning on June 30, 2000 and thereafter with each royalty report provided for in Paragraph 5.9, LICENSEE shall submit to ASU a progress report covering LICENSEE'S activities related to the testing and development of all LICENSED PRODUCTS~ along with the obtaining of the governmental approvals necessary for marketing of LICENSED PRODUCTS. The LICENSEE shall make these progress reports for each LICENSED PRODUCT until the first commercial sale of that LICENSED PRODUCT occurs in the United States. |
| 9.2. | The progress reports submitted under Paragraph 9.1 shall include, but not be limited to, the following topics: |
| • |
summary of work completed |
| • |
key scientific discoveries |
| • |
summary of work in progress |
| • |
current schedule of anticipated events or milestones |
| 9.3. | At the request of ASU from time to time, LICENSEE will provide information necessary |
-12-
|
to confirm the large/small entity status (as defined by the United States Patent and Trademark Office) of itself and its SUB-LICENSEES and AFFILIATES. |
| 9.4. | The LICENSEE shall report to ASU the date of first commercial sale of a LICENSED PRODUCT in each country. |
| 9.5. | LICENSEE shall keep, and it shall cause its SUB-LICENSEES to keep, accurate records in sufficient detail to enable the payments due under Article 5 to be determined for a period of 3 years following the end of the accounting period to which the information pertains. Upon the request of ASU, LICENSEE and its SUB-LICENSEES shall permit an independent certified public accountant selected by ASU to have access, once in each calendar year during regular business hours and upon reasonable notice to LICENSEE, to those records of LICENSEE and its SUB-LICENSEES as may be necessary or desirable to verify the accuracy of the reports made during the previous calendar year. Should the audit reveal a discrepancy of more than 5% between the payment reported and the payment actually due to ASU, LICENSEE shall pay all fees and expenses incurred in conducting the audit; otherwise ASU shall pay the fees and expenses incurred in conducting the audit. |
10. WARRANTY
ASU warrants and represents that except for the possible government interest disclosed above, it has the full right and power to grant the license described in Article 2, that it will take no action to negate this right and power and shall take all actions within its control to maintain this right and power and that it has no knowledge of any outstanding undisclosed agreements, assignments, or encumbrances inconsistent with the provisions of this Agreement other than as expressly set forth herein. ASU makes no other representation or warranty, express or implied, and ASU assumes no liability with respect of any infringement of any patent or other right of third parties due to LICENSEE's activities under the LICENSE granted hereunder and ASU assumes no liability with regard to any claim, specious or otherwise, arising out of alleged side effects or any other alleged performance defect arising out of the use or misuse of the LICENSED PRODUCTS.
11. PATENT ENFORCEMENT
| 11.1. | If at any time during the TERM of this Agreement either party shall become aware of any infringement or threatened infringement of any of ASU's PATENT RIGHTS, such party shall give immediate notice of it to the other party. If the infringement relates to the TECHNOLOGY, then LICENSEE shall have the first right to settle any alleged infringement of ASU's PATENT RIGHTS. ASU shall have the first right to settle any alleged infringement of ASU's PATENT RIGHTS that does not relate to the TECHNOLOGY. Each party shall give reasonable assistance to the other party in connection with settling any alleged infringement and shall have the right to join in any infringement or enforcement action at its own expense. |
| 11.2. |
If the party having the first right to take action under Section 11.1 is not able or willing to take action against an infringer as set forth above, the other party shall have the right at any |
-13-
|
time following one hundred twenty (120) days of receipt of notice of the alleged infringement, to at its or their own expense take action against the infringer. ASU shall permit, if legally necessary, the use of its name and shall execute any documents and do any acts as may be reasonably necessary for the purpose of taking action. Any recovery received by the party taking action against an infringer pursuant to this Paragraph shall be retained for the benefit of such party, provided that in the case of LICENSEE taking such action, royalties specified in Article 5 shall be paid to ASU on that portion of any recovery remaining after reimbursement of all of LICENSEE'S expenses hereunder and in the case of ASU taking such action, ASU shall pay to LICENSEE the amount of any damages for injury to LICENSEE or its SUBLICENSEES resulting from the infringement. |
| 11.3. | If LICENSEE, its AFFILIATES or SUB-LICENSEES must pay royalties or license fees in any country to third parties under one or more valid claims of a dominant patent to enable LICENSEE, its AFFILIATES or SUB-LICENSEES to use the inventions of the ASU's PATENT RIGHTS, those payments shall be credited against LICENSEE's royalty obligations to ASU hereunder for sales in that country where a valid claim of a dominant patent exists to the extent of payments of royalties or license fees actually made to third parties by LICENSEE, its AFFILIATES and SUB-LICENSEES. |
| 11.4. | After an initial determination by a court or tribunal that a claim or claims of any of ASU's PATENT RIGHTS is invalid, LICENSEE shall place all royalties due by virtue of such ASU PATENT RIGHT in an interest-bearing escrow account until a decision by a court of last resort. If the court of last resort reverses the initial determination, LICENSEE shall cause to be paid to ASU all amounts in escrow plus accrued interest within thirty (30) days after receipt of the determination of the court of last resort. If the court of last resort upholds the initial determination, LICENSEE shall receive all amounts in escrow, plus accrued interest. |
| 11.5. | Each party agrees to use its best efforts whenever a protective order is to be entered with a court of competent jurisdiction, to have the order permit at least one counsel from each party access to information provided under the protective order without restriction. |
12. COMMUNICATION
Any payment, notice, or other communication required or permitted to be made or given to either party pursuant to this Agreement shall be sufficiently made or given on the date of mailing if sent to the party by certified or registered mail, postage prepaid, addressed to it at its address set forth or to such other address as it shall be designated by written notice to the other party as follows:
In the case of ASU:
Office of Technology Collaborations & Licensing
Office of the Vice Provost for Research
Arizona State University
P. 0. Box 873511
Tempe, AZ 85287.3511
-14-
USA
Attn: Director
In the case of LICENSEE:
Seattle Genetics, Inc.
22215 26th Avenue, SE
Bothell, Washington 98021
Attn: Chief Executive Officer
13. ASSIGNMENTS
This Agreement shall not be assignable by either party without the prior written consent of the other party except to an AFFILIATE or to a successor in ownership of all or substantially all of the business assets to which this Agreement pertains and then only if such successor shall expressly assume in writing the performance of all the terms and conditions of this Agreement which are to be performed by the assigning party.
14. TECHNICAL ASSISTANCE
| 14.1. | At LICENSEE's written request, ASU shall: |
| 14.1.1. | permit representatives from LICENSEE and its SUB-LICENSEES to visit the facilities of ASU for the purpose of personally observing the practice and testing of TECHNOLOGY or the production of LICENSED PRODUCTS, and |
| 14.1.2. | arrange for its or its AFFILIATES' representatives to visit the facilities of LICENSEE, or its SUB-LICENSEES as may be designated by LICENSEE to provide LICENSEE, or its SUB-LICENSEES any technical assistance and advice as LICENSEE, and its SUB-LICENSEES may reasonably require in connection with the production, packaging, inspecting, and testing of TECHNOLOGY and the LICENSED PRODUCTS or the LICENSED METHODS. |
| 14.2. | LICENSEE shall give ASU reasonable prior notice of the visits or required assistance referred to in Paragraphs 14.1.1 and 14.1.2 above and the visits shall be of reasonable duration and made at reasonable times during regular business hours. LICENSEE and its SUB-LICENSEES shall bear the entire cost of the visits made pursuant to Paragraph 14.1.1 and shall promptly reimburse ASU and its AFFILIATES for all reasonable salary, travel, and other expenses actually incurred by ASU and its Affiliates' representatives in the course of the visits made to LICENSEE's and its SUB-LICENSEES' facilities pursuant to Paragraph 14.1.2. |
15. PATENT MARKING
-15-
The LICENSEE shall mark all LICENSED PRODUCTS made, used or sold under the terms of this Agreement, or their containers, in accordance with the applicable patent marking laws.
16. TERMINATION
| 16.1. | Material failure by ASU or LICENSEE to comply with any of the material obligations and conditions contained in this Agreement (a "Default") shall entitle the non-Defaulting party to give to the party in Default, written notice requiring it to cure the Default. If the Default is not cured or, if in the non-Defaulting party's judgment, substantial steps have not been taken to cure the Default, within 90 days after the receipt of the notice by the Defaulting party, the non-Defaulting party shall be entitled (without prejudice to any of its other rights conferred on it by this Agreement) to terminate this Agreement in whole or in part by giving notice to take effect immediately upon receipt by the party in Default; provided, however, that with respect to a Default by LICENSEE under Paragraph 8.6., ASU's termination right hereunder shall only apply with respect to the LICENSED PRODUCT(S) which is the subject of the Default. If the parties disagree as to the existence of any Default, such matter shall be resolved prior to any termination hereunder by arbitration to be conducted in Maricopa County, Arizona upon 60 days prior written notice under the then prevailing rules of the American Arbitration Association. The right of either party to terminate this Agreement shall not be affected in any way by its waiver of, or failure to take action with respect to, any previous Default. |
| 16.2. | if one of the parties shall voluntarily or involuntarily go into liquidation or bankruptcy, make an assignment for the benefit of creditors, or have a receiver or a trustee appointed for its properties, the other party shall be entitled to terminate this Agreement immediately upon written notice to that party. |
| 16.3. | LICENSEE may terminate this Agreement with respect to such LICENSED PRODUCT or ASU PATENT RIGHT upon 30 days prior written notice with no further obligation to ASU except for the payment of any fees which came due or royalties accrued up until the date of termination. |
| 16.4. | Upon any termination of this Agreement, any SUB-LICENSEE then in good standing shall have the right to continue as a licensee under the relevant rights granted to it hereunder after agreeing in writing to directly assume all relevant obligations of LICENSEE hereunder. |
17. RIGHTS AND OBLIGATIONS FOLLOWING TERMINATION
| 17.1. | Termination of this Agreement, by expiration or otherwise for any reason, shall be without prejudice to: |
| • |
the rights and obligations provided for in Paragraph 7.3; |
| • |
ASU's right to receive all payments and royalties due and accrued and unpaid on the effective date of the termination; |
-16-
| • |
the rights and obligations provided for in Article 10, Article 17 and Article 28; and |
| • |
any other remedies which either party may have under law or equity. |
| 17.2. | Following any termination but not the expiration of this Agreement, LICENSEE and its SUB-LICENSEES, may sell, in accordance with the terms of this Agreement, any affected LICENSED PRODUCT which was in process of manufacture or finished on the effective date of the termination, but, with respect to these sales, LICENSEE shall continue to be bound by all of its obligations under this Agreement, including the obligation to render quarterly reports covering sales in accordance with the provisions of Article 9 and the obligation to pay royalties at the rates set forth in Article 5. The right of each party, subsequent to the loss of its license or sub-license upon termination of this Agreement, to challenge the validity or alleged infringement under which a license or sub-license is granted, shall not be prejudiced by reason of the prior existence of this Agreement. |
18. INSURANCE AND INDEMNIFICATION
| 18.1. | LICENSEE shall at all times comply, through insurance or self-insurance, with all statutory worker's compensation and employers' liability requirements covering all employees with respect to activities performed under this Agreement. In addition, LICENSEE shall maintain, from the initiation of human trials, if applicable, and for so long as LICENSEE customarily maintains insurance for its other products, Comprehensive General Liability Insurance, including Products Liability Insurance, with reputable and financially secure insurance carriers to cover the activities of LICENSEE and its SUB-LICENSEES. This insurance shall provide minimum limits of liability of $2,000,000 and shall include the State of Arizona, the Arizona Board of Regents, Arizona State University and their Regents, officers, employers, students and agents as additional insureds. This insurance shall be written to cover claims made during or after the expiration of this Agreement. At ASU's request, LICENSEE shall furnish a Certificate of Insurance evidencing primary coverage and requiring 30 days prior written notice of cancellation or material change to ASU. LICENSEE shall advise ASU, in writing, that it maintains excess liability coverage over primary insurance for at least the minimum limits set forth above. All insurance of LICENSEE shall be primary coverage; insurance of ASU or the State of Arizona shall be excess and noncontributory. |
| 18.2. | LICENSEE agrees to indemnify, hold harmless and defend the State of Arizona, the Arizona Board of Regents, ASU, its officers, employees and agents; the sponsors of the research that led to the TECHNOLOGY; and the INVENTOR of the patents and patent application included in ASU's COLLECTIVE PATENT RIGHTS (collectively, the INDEMNITEES) against any and all claims, suits, losses, damages, costs, fees, and expenses resulting from or arising out of exercise of rights granted under this Agreement; provided, however, that LICENSEE shall have no obligation to indemnify any INDEMNITEE for negligence or willful misconduct or breach of any representation contained in this Agreement by such INDEMNITEE. |
| 18.3. |
ASU shall promptly notify LICENSEE in writing of any claim or suit brought against ASU in respect of which ASU intends to invoke the provisions of Paragraph 18.2. LICENSEE |
-17-
|
will keep ASU informed on a current basis of its defense of any claims pursuant to Paragraph 18.2. |
19. FORCE MAJEURE
The parties shall not be liable for failure or delay upon fulfillment of all or part of this Agreement, directly or indirectly owing to acts of Nature, Governmental orders or restriction, war, warlike condition, revolution, riot, looting, strike, lockout, fire, flood, or any other cause or circumstances beyond the parties' control including the disability or death of an INVENTOR.
20. LATE PAYMENTS
In the event royalty payments, re-billings or fees are not received by ASU when due, the LICENSEE shall pay to ASU interest charges at a rate of ten (10) percent per annum. Interest shall be calculated from the date payment was due until actually received by ASU.
21. WAIVER
No waiver by either party to this Agreement of any breach or default of any of the covenants or agreements set forth in this Agreement shall be deemed a waiver as to any subsequent and/or similar breach or default.
22. COMPLIANCE
LICENSEE shall manufacture LICENSED PRODUCTS in accordance with applicable US law.
23. GOVERNING LAWS INCLUDING ARIZONA PUBLIC RECORDS LAW
THIS AGREEMENT SHALL BE INTERPRETED AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF ARIZONA, but the scope and validity of any patent or patent application shall be governed by the applicable laws of the country of such patent or patent application.
24. REPRESENTATIONS
Each party represents that it is authorized to enter into this Agreement and that in the due performance of its obligations it would not be acting in violation of any outstanding obligation, contractual or otherwise, that may be owed by that party to any third party.
25. PREFERENCE FOR UNITED STATES INDUSTRY
Because this Agreement grants the exclusive right to use or sell the TECHNOLOGY in the United States, the LICENSEE agrees that any products embodying this TECHNOLOGY or produced through the use of the TECHNOLOGY will be manufactured substantially in the United States.
26. FOREIGN GOVERNMENT APPROVAL OR REGISTRATION
-18-
If the law of any nation requires that this Agreement or any associated transaction be either approved or registered with any governmental agency, the LICENSEE shall assume all legal obligations to do so.
27. EXPORT CONTROL LAWS
The LICENSEE shall observe all applicable United States and foreign laws with respect to the transfer of LICENSED PRODUCTS and related technical data to foreign countries, including, without limitation, the International Traffic in Arms Regulations (ITAR) and the Export Administration Regulations.
28. MISCELLANEOUS
| 28.1. | This Agreement will not be binding upon the parties until it has been signed below on behalf of each party; it shall then be effective as of the EFFECTIVE DATE. No amendment or modification shall be valid or binding upon the parties unless made in writing and signed by each party. |
| 28.2. | This Agreement embodies the entire understanding of the parties and shall supersede all previous communications, representations, or undertakings, whether verbal or written, between the parties hereto relating to its subject matter. |
| 28.3. | LICENSEE shall have no right to use the name or other designation of the Arizona Board of Regents or Arizona State University or the INVENTOR in connection with any sale or promotion of LICENSED PRODUCT without the express written consent of the Arizona Board of Regents, ASU or the INVENTOR, respectively. |
| 28.4. | If any provision of this Agreement shall be held to be invalid, illegal, or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired. |
| 28.5. | The headings of the articles are inserted for convenience of reference only, and are not intended to influence the interpretation of this Agreement. |
| 28.6. | ASU is a public institution and only those obligations imposed upon ASU which can be lawfully undertaken by the Board of Regents in accordance with its legislative charter shall be enforceable. |
| 28.7. |
LICENSEE agrees that the personnel of LICENSEE will not for any purpose be considered employees or agents of ASU and that LICENSEE assumes full responsibility for the actions of its personnel while performing services under this Agreement, and shall be solely responsible for their supervision, daily direction and control, payment of salary (including withholding income taxes and social security), worker's compensation and disability benefits. ASU agrees that the personnel of ASU will not for any purpose be considered employees or agents of LICENSEE and that ASU assumes full responsibility for the actions of its personnel while performing services under this Agreement, and shall |
-19-
|
be solely responsible for their supervision, daily direction and control, payment of salary (including withholding income taxes and social security), worker's compensation and disability benefits. |
| 28.8. | The parties agree to comply with all applicable state and federal laws, rules, regulations and executive orders as to equal employment opportunity, nondiscrimination and affirmative action. |
| 28.9. | This Agreement is subject to Section 38-5 11, Arizona Revised Statutes. |
| 28.10. | In the event of a dispute under this Agreement, the parties agree to use arbitration to the extent required under Sections 12-1518 and 12-133, Arizona Revised Statutes. |
| 28.11. | To the extent required by Section 35-214, Arizona Revised Statutes, LICENSEE agrees to retain all books, accounts, reports, files and other records of LICENSEE relating to this Agreement and make those records available at all reasonable times for inspection and audit by ASU or the Auditor General of the State of Arizona, or their agents, during the terms of and for a period of five (5) years after the completion of this Agreement. |
-20-
IN WITNESS WHEREOF, both ASU and LICENSEE have executed this Agreement, in duplicate originals, by their respective officers hereunto duly authorized, as of the EFFECTIVE DATE.
| ARIZONA BOARD OF REGENTS a body corporate of the State of Arizona acting for ARIZONA STATE UNIVERSITY ("ASU") | SEATTLE GENETICS, INC. ("LICENSEE") | |||||||
| By: |
/s/ Alan M. Poskanzer |
By: |
/s/ H. Perry Fell |
|||||
| Name: | Alan M. Poskanzer, Ph.D. | Name: | H. Perry Fell, Ph.D., M.B.A. | |||||
| Title: |
Director Office of Technology Collaborations & Licensing |
Title: | President & CEO | |||||
| Date: |
February 4, 2000 |
Date: |
2/3/2000 |
|||||
-21-